Welcome to the Hoang Lab
Meet Our PI and Our Team

Thanh Hoang
Located at the University of Michigan Kellogg Eye Center, our lab studies fundamental biology of neurodevelopment and regeneration in the brain and retina. We employ large-scale multiomic and genetic approaches in both mouse and human systems. Our ultimate goal is to translate these understandings into therapeutic strategies for treating neurodegenerative diseases.

Research Focus
Regeneration of neurons through cell reprogramming

Investigating cell type specification during neurodevelopment

Modulating the cell interactions for neuroprotection
Featured Publications
Science Advances | 2024
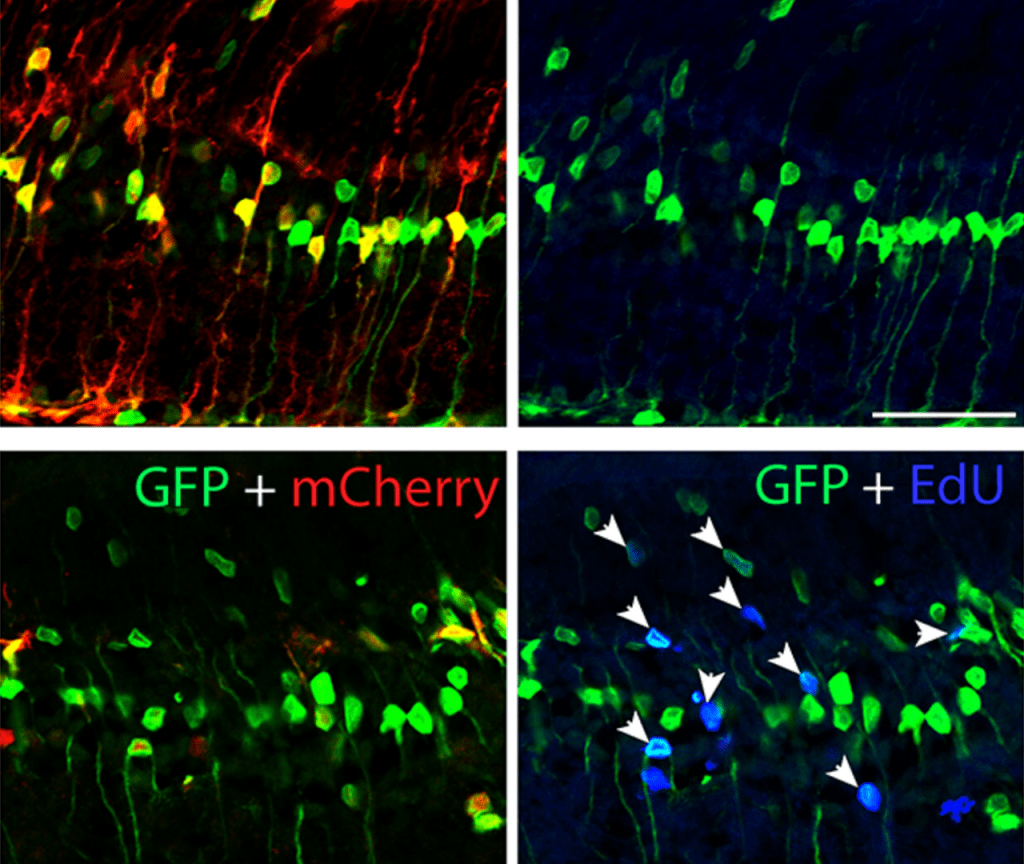
Robust reprogramming of glia into neurons by inhibition of Notch signaling and nuclear factor I (NFI) factors in adult mammalian retina
Nguyet Le, Trieu-Duc Vu, Isabella Palazzo, Ritvik Pulya, Yehna Kim, Seth Blackshaw, Thanh Hoang
Suppressing Notch signaling (via Rbpj or Notch1/2 disruption) reprograms mature Müller glia into bipolar- and amacrine-like retinal neurons. Combined loss of Rbpj and Nfia/b/x converts nearly all glia to neurons, while Yap-driven proliferation further enhances neurogenesis. Notch/NFI pathways act in parallel to inhibit glial neurogenic competence, revealing targets for retinal regenerative therapies.

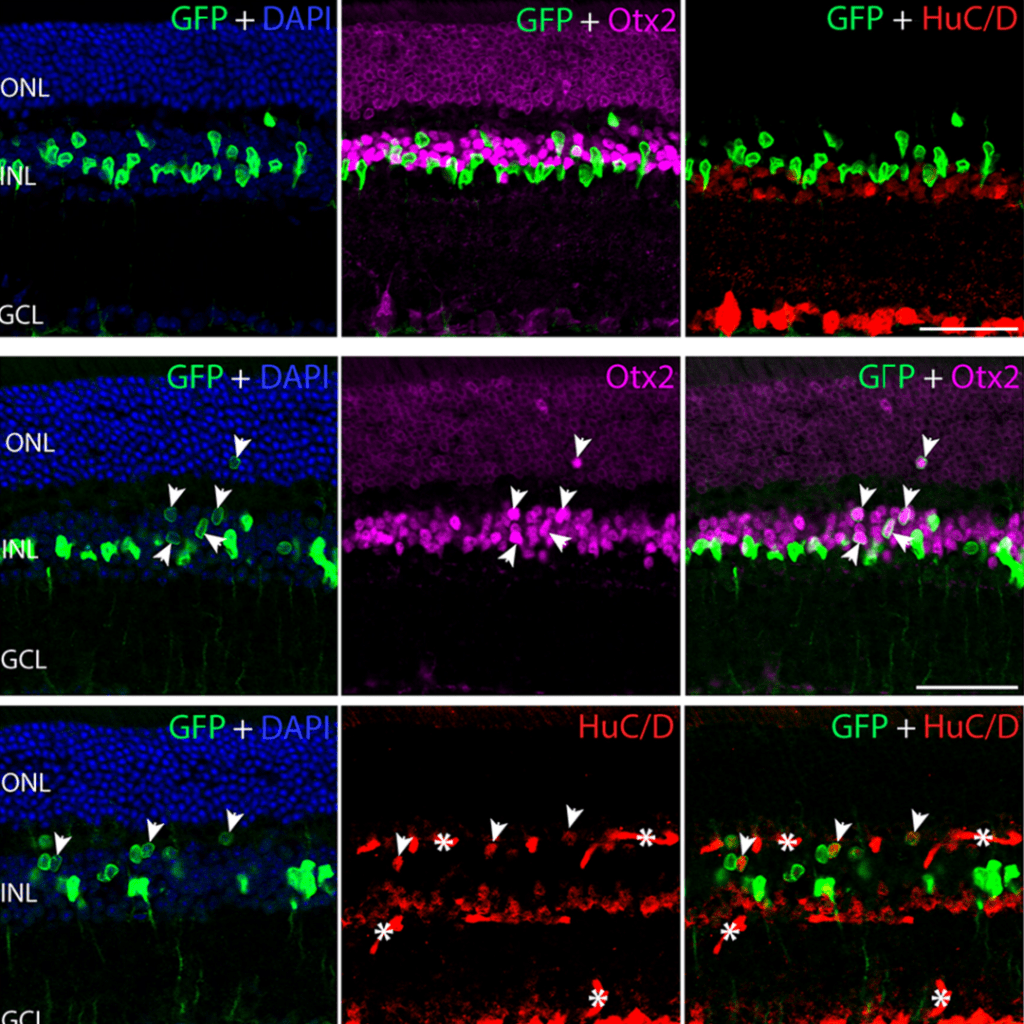
Nature | 2023
Ptbp1 deletion does not induce astrocyte-to-neuron conversion
Thanh Hoang, Dong Won Kim, Haley Appel, Manabu Ozawa, Sika Zheng, Juhyun Kim & Seth Blackshaw
Genetic disruption of Ptbp1 in astrocytes using rigorous lineage tracing and single-cell RNA sequencing revealed no evidence of astrocyte-to-neuron conversion, contradicting prior claims. The observed “conversion” in earlier studies likely resulted from artifacts such as leaky neuronal labeling, emphasizing the need for stringent controls in reprogramming research.
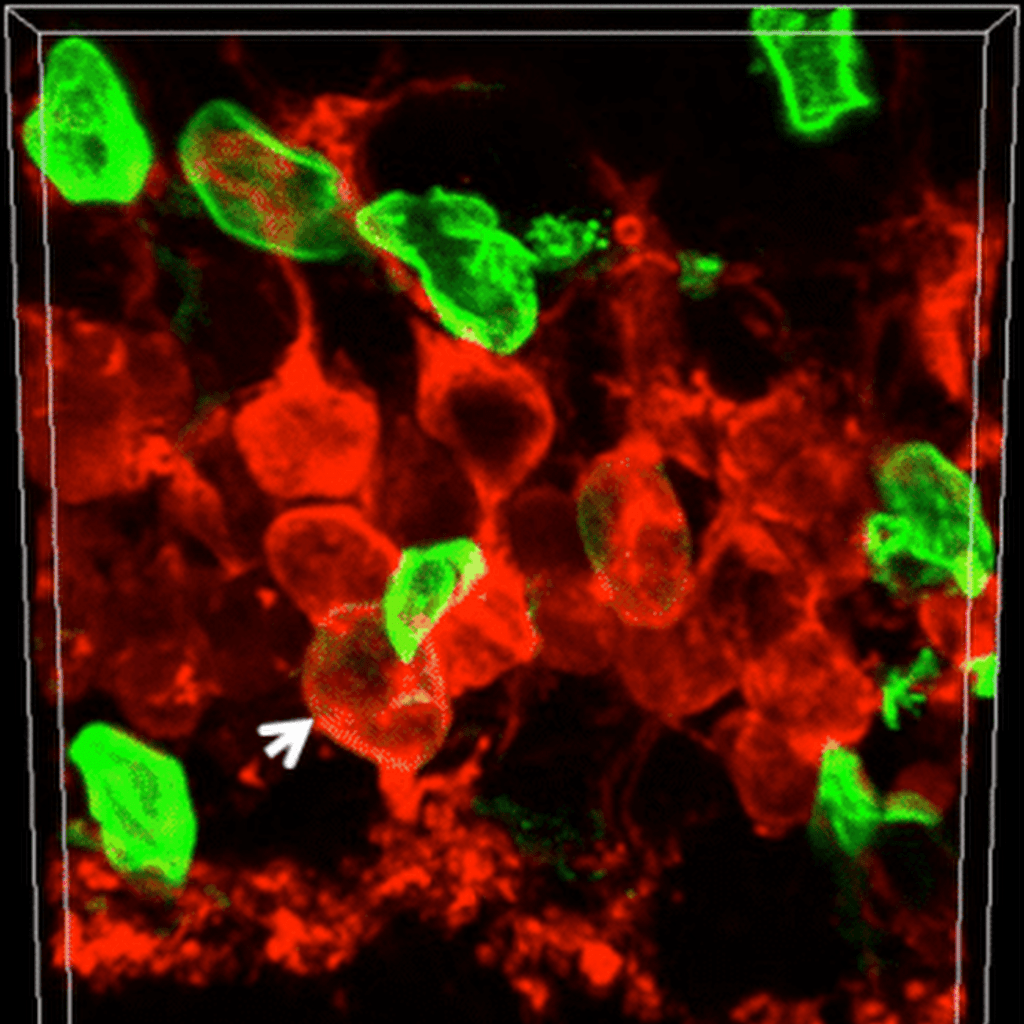
Science | 2020
Gene regulatory networks controlling vertebrate retinal regeneration
Thanh Hoang, Jie Wang, Patrick Boyd, Fang Wang, Clayton Santiago, Lizhi Jiang, Sooyeon Yoo, Manuela Lahne, Levi J. Todd, Meng Jia et al.
Retinal Müller glia in zebrafish and chick reprogram into neurogenic progenitors after injury, while mammalian Müller glia remain quiescent due to a suppressive gene network. Disrupting nuclear factor I transcription factors unlocks neurogenic potential in mice, suggesting therapeutic strategies for retinal repair in degenerative diseases.